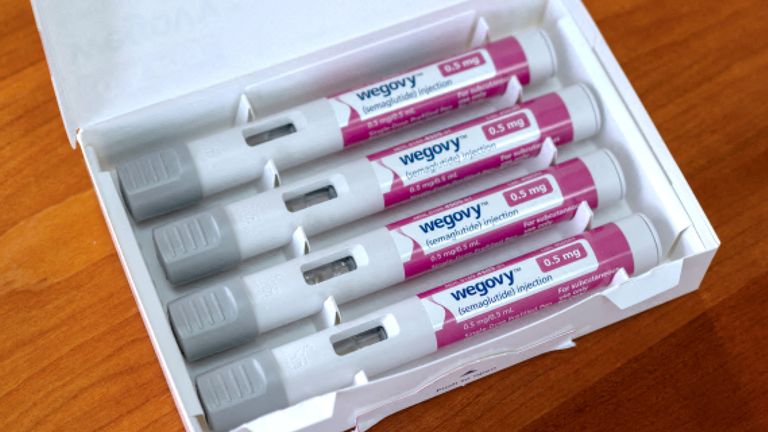
A weight-loss jab has been approved by the UK’s medicines regulator for use in preventing heart attacks and strokes in overweight or obese adults.
Wegovy, which is the brand name for the drug semaglutide, had already got the green light for weight management in those with obesity.
The Medicines and Healthcare products Regulatory Agency (MHRA) has now allowed it to be used in helping overweight or obese people cut their risk of heart problems.
Wegovy is the first weight-loss medication to be approved in the UK as a preventative method for “established cardiovascular disease”.
It can be prescribed to people who have a body mass index (BMI) score of 27 or above and have already been diagnosed with cardiovascular disease – a term which describes conditions relating to the heart or blood vessels.
Wegovy, made by Novo Nordisk, typically works by making people feel fuller and less hungry.
It does so by mimicking the GLP-1 hormone which is used in regulating blood sugar levels and enhancing insulin secretion. This reduces the amount of glucose, or sugar, produced by the liver.
By cutting the amount of sugar produced, it slows down how quickly food is digested.
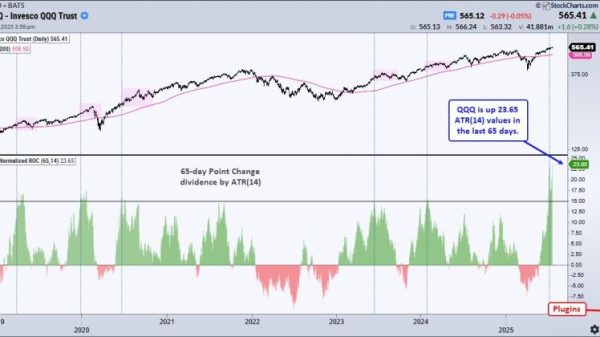
Heart attack or stroke risk cut by a fifth – study
The approval comes as a new trial, involving 17,600 people, found that taking it as an injection once a week, for up to five years, can lower a person’s risk of a major cardiovascular event – such as a heart attack or stroke – by 20%.
While the National Institute for Health and Care Excellence (NICE) is still to approve the treatment’s use, Professor Sir Stephen Powis, national medical director for NHS England, is feeling positive.
He said it could “help reduce cardiovascular risks for high-risk patients, potentially preventing heart attacks and strokes, and giving more people the chance of a healthier future”.
The MHRA’s Shirley Hooper called it “an important step forward in tackling the serious health consequences of obesity”.
She also said she is assured “the appropriate regulatory standards of safety, quality and effectiveness” have been met.
There have been previous challenges around the supply of the medication, with Novo Nordisk warning earlier this year there were supply constraints, and that it would be focusing on supplying to “those with the highest ongoing need”.
However, for the NHS, the company states there is “a protected supply”, while the non-NHS supply will remain “constrained and limited for the foreseeable future”.